Describe the Evidence for the Existence of Electrons
A Princeton-led experiment suggests that a controversial theory that the electron is composed of two particles could be true. Describe the evidence for the existence of protons.
Structure Of Atom Discovery Of Electrons Protons And Neutrons Open Teaching Project
What is the evidence for the existence of two photosystems.
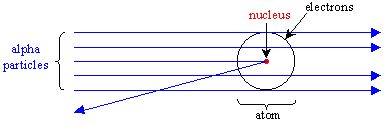
. Because electrons are so light low mass their de Broglie wavelengths are much much larger than the wavelengths of the nucleus and therefore even when the electrons and nucleus share the same center of mass the electron is much more diffuse and the nucleus much more concentrated. In the late 19th century scientists were experimenting with vacuum tubes and electricity. They threw energetic electrons to a gas tube in their original experiment they used mercury and they found very sudden drops in the transmitted electron flux at concrete energies.
Section 81 The Smallest Pieces Solution. Describe how the wave properties of the electron are taken into account in the current model of the atom. Describe the components of an atom and discuss the experimental evidence and theory that supports their existence charge and location.
A Explain what is meant by the ground state of an atom and describe the process that is taking place in the atoms emitting photons. From this diagram it is evident that electrons emit photons of only certain wavelengths are emitted which concurs w. Describe the three phases of the Calvin Cycle and how the products of the light-capturing reactions participate in the process.
When an electron in an hydrogen atom is somehow excited to a higher level say n1 to n2 it wants to return back to its level of origin in this case the ground state. He later discovered protons as well. As a result of these collisions photons of various frequencies are emitted by some of the atoms.
None of the electron activity which is evoked by the actions of the sodiumpotassium pump an attribute of the physiology of neurons would take place. B number of neutrons in the nucleus of the atom. First evidence scientist who were studying electricity not atomic structure.
The correct answer was given. In this model negative electrons are scattered throughout a sea of positive charge. Electrons excited by the absorption of light in photosystem I are transferred to iron-sulfur electron acceptors and therefore must be replaced.
45 Describe the difference in the atomic number and number of electrons in typical hydrogen and helium atoms and their chemical effects. Answer 1 of 12. Describe the number and location of orbitals and electrons using quantum numbers orbital diagrams and electron configurations.
In 1911 Ernest Rutherford discovered the nucleus. Rutherford thought that electrons randomly orbit the nucleus. Calculate the wavelength of particles given mass and velocity.
Also the negative charge of electrons must be compensated by the positive charge. A new discovery led by Princeton University researchers could upend our understanding of how electrons behave under extreme conditions due. George is a dalmatian puppy.
Electrons with a range of kinetic energies strike atoms of a particular element which are in their ground state. These experiments showed such things like if you added a small amount of gas into a vacuum tube and ran an electric current through it it would glow. Describe experimental evidence for the wave nature of electrons.
LO 81 Describe evidence for the existence of atoms and their role in the composition of the chemical elements. The act of believing or deciding not to is proof that electrons exist. Historically the first strong evidence for the existence of discrete orbitals was the Franck-Hertz experiment.
Begingroup Greg wote They do not exist Electrons exist and they occupy regions with probabilities defined by equations. Using a cathode ray tube with holes in the cathode he noticed that there were rays traveling in the opposite direction from the cathode rays. Without electrons you wouldnt believe or not believe anything because your neurons would be dead-wood in your skull.
He proposed the plum pudding model of the atom. LO 81 Describe evidence for the existence of atoms and their role in the composition of the chemical elements. Recall that successive ionisation energies provide evidence for the existence of quantum shells and the group to which the element belongsfirst ionisation energy of successive elements provides evidence for electron subshells.
The most convincing evidence for the existence of electrons cane from The most convincing evidence for the existence of electrons cane from Answers. Rays must have come from teh atoms of the cathode most of te atoms in the air had been pumped out of the tube. Orbital is a word that can be used to describe that.
Describe what happens to light that allows you to see georges black and white coat. Section 81 The Smallest Pieces 8 In todays periodic table elements are arranged in order of a number of protons in the nucleus of the atom. An electron has a negative charge.
In 1886 Eugene Goldstein 18501930 discovered evidence for the existence of this positively charged particle. These regions may have different spatial functions. He called these canal rays and showed that they were composed of positively charged particles.
Electrons are discovered by using cathode rays. Such an electron will emit photons of certain wavelength as they return to. New evidence for electrons dual nature found in a quantum spin liquid.

Charged Particles In Matter Discovery Charge Of An Electron Examples

No comments for "Describe the Evidence for the Existence of Electrons"
Post a Comment